
New research from a team of scientists co-led by Dr. Runze Yu from HPSTAR has revealed an intricate interplay between atomic-ordering manipulation and the dynamic catalytic evolution of catalysts within a triple-perovskite platform, Ba3MRu2O9 (M=Fe, Co). The study is published in Applied Catalysis B: Environment and Energy.
Perovskite-type compounds are well-known for their compositional versatility and structural tunability, making them a cornerstone in the design of advanced catalysts for a wide range of electrocatalytic applications. However, most simple perovskite oxides are suboptimal catalysts for the hydrogen evolution reaction (HER), due to a high energy barrier for hydrogen adsorption. This inefficiency arises from the crystallographically defined B-site arrangements, which create excessively wide interatomic spacings, disrupting the Tafel mechanism. As such, the targeted manipulation of cation ordering is essential for developing high-performance perovskite catalysts for sustainable hydrogen production systems.
In recent years, the strategic engineering of atomic ordering has emerged as a transformative approach to optimizing catalysts, allowing for the systematic tuning of catalytic properties via structural modifications. In this study, the team propose a novel approach to modulate the atomic ordering in Ru-based triple perovskites Ba₃MxRu₂₋ₓO₉ (M = Fe, Co) as a conceptual framework to improve HER catalytic kinetics.
Using a chemical pressure engineering approach, three distinct perovskite models—Ba3FeRu2O9 (disordered), Ba3Fe1.5Ru1.5O9 (half-ordered) and Ba3CoRu2O9 (highly ordered)—were synthesized. Multimodal characterization and computational modeling revealed that, for Ba3FeRu2O9, an in-situ heterogeneous architecture formed through controlled iron reduction activates an interfacial built-in electric field (BEF). This BEF modulates the surface electronic configuration, driving it closer to the optimal catalytic potential while maintaining high stability.
As a representative example, Ba3FeRu2O9 exhibits exceptional outstanding HER performance, achieving an ultralow overpotential of 24 mV at 10 mA cm-2 and a Tafel slope of 28 mV dec-1, while maintaining remarkable operational stability over 600 h and withstanding 80,000 electrochemical cycles. This study highlights that engineering the degree of atomic ordering allows for the design of template-like pre-catalysts that can precisely regulate dynamic catalytic processes, providing a key strategy for developing robust, high-performance electrocatalytic systems.
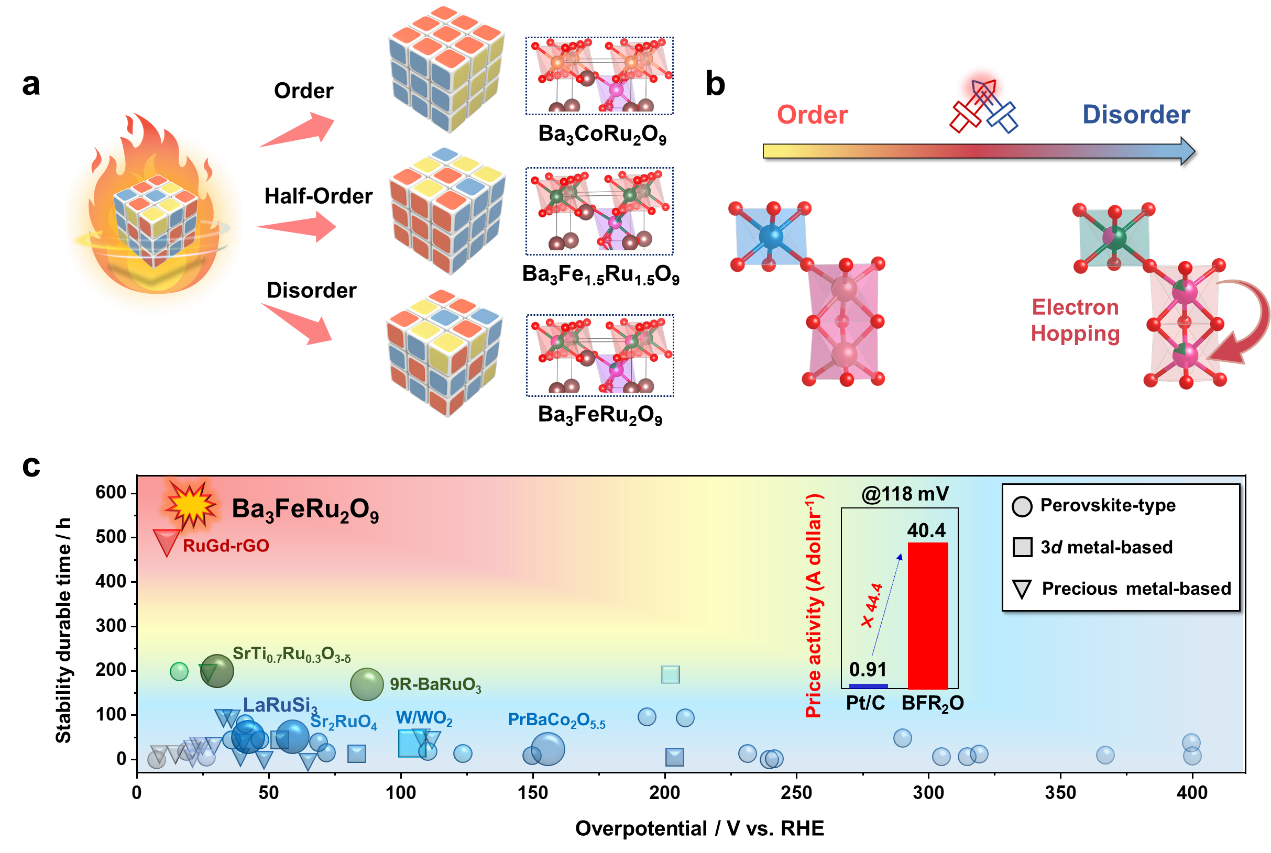
Caption: (a) Schematic of the design of perovskite crystals (b) Schematic diagram of polyhedral primitive for Ba3CoRu2O9 (left) and Ba3FeRu2O9 (right), which illustrate the electron hopping through adjacent octahedron via super-exchange interaction. (c) Overpotentials and the stability durable time of Ba3FeRu2O9 and other recently reported high-performance HER catalysts.
开发高效、稳定的析氢反应电催化剂是实现绿色氢能源的关键。铂基催化剂性能优异但成本高昂,而大多数钙钛矿氧化物虽结构可调,但其固有的氢吸附能垒过高,限制了本征催化活性。如何在原子尺度上精确调控催化剂的结构,以同时实现高活性和高稳定性,是当前该领域面临的重大挑战。北京高压科学研究中心(HPSTAR)于润泽研究员团队在原子级有序度调控提升电催化制氢性能方面取得重要突破。研究团队以三钙钛矿氧化物Ba3MxRu2-xO9(M=Fe, Co)为模型,通过在八面体基元([MO6]与[M2O9])中精确构建从有序到无序的晶体学梯度,成功揭示了原子排列有序度如何通过调控电子结构与诱导界面自重构,从而决定性影响催化剂的活性和稳定性。其中,高度无序的Ba3FeRu2O9在析氢反应过程中通过铁的自牺牲还原,原位构建了具有强内置电场的异质界面。该电场显著优化了反应中间体(H和OH)的吸附/脱附动力学,从而展现出卓越的析氢反应性能。该研究成果为设计新一代高性能电催化材料提供了全新的思路和范式。相关结果于近期以“Manipulating atomic order-disorder in octahedral building blocks ([MO6]/ [M2O9]) for advanced hydrogen evolution electrocatalysis”为题发表于催化材料顶刊Applied Catalysis B: Environment and Energy。