A new graphitic carbon nitride made under pressure - Dr. Huiyang Gou
AUGUST 14, 2017
Nitrogen-bearing, carbon-rich materials are known to have multiple functionalities and diverse applications. Graphitic carbon nitride (g-C3N4) is the most common C-N phase studied, but several other phases with varying compositions and properties have been predicted. A group of scientists led by Huiyang Gou of HPSTAR and Timothy Strobel of Geophysical lab performed high-pressure experiments on linear dicyanoacetylene (C4N2) using a diamond anvil cell, in which a pressure-induced reaction process of was uncovered. Discrete linear C4N2 molecules were found to polymerize into a disordered extended network without significant change to the bulk composition. The results were published in Chemistry of Materials.
C4N2 is a linear molecule with alternating triple and single bonds (N≡C-C≡C-C≡N) that can burn with a flame temperature of more than 5000 K. This high-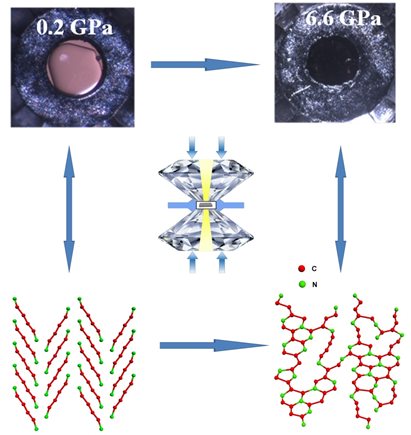 energy state represents a novel starting point for the synthesis of new carbon nitride extended networks with an interesting C:N stoichiometry in-between typical deposition-based syntheses (nitrogen doping) and systems like graphitic C3N4.
energy state represents a novel starting point for the synthesis of new carbon nitride extended networks with an interesting C:N stoichiometry in-between typical deposition-based syntheses (nitrogen doping) and systems like graphitic C3N4.
The team found that C4N2 polymerizes into a disordered carbon/nitrogen network that can be recoverable at ambient conditions. Its local structure, composition and chemical bonding were established using a variety of optical and X-ray scattering methods. Simulations of the reaction process conducted by Li Zhu and Duck Young Kim indicate that the pressure-induced polymerization initiates between the shortest N···C distances within a single two-dimensional “sheet” of molecules within the crystal. The polymerization proceeds within individual sheets through cycloaddition reactions producing predominantly 5- and 6-membered heterocyclic rings, giving the overall structure a tendency to propagate in two dimensions.
The high-pressure behavior of C4N2 elucidates the nature of chemical bonding, reaction mechanisms, and the atomic structure of reaction products, which advances the general understanding of carbon nitride materials and provides fundamental contributions for realizing new diamond-like carbon nitride materials
Other team members included Haw-Tyng Huang, Arani Biswas, Derek Keefer, Jordan Lerach and John Badding of The Pennsylvania State University; Brian Chaloux and Albert Epshteyn of the Naval Research Laboratory; Clemens Prescher of the University of Chicago; Liuxiang Yang and Matthew Ward of Carnegie; Shengnan Wang and Artem Oganov of Stony Brook University.
Caption: C4N2 polymerizes into a disordered carbon/nitrogen network under compression.
炭素材料以及富碳-碳氮材料具有独特的电子结构和优异的化学稳定性,广泛应用于光催化产氢、水氧化、有机物降解、光合成以及二氧化碳还原等,因此在能源和材料相关领域备受重视。碳氮相图中最稳定的是石墨相g-C3N4,目前被认为是最有可能代替碳材料的新型应用功能材料之一。北京高压科学研究中心的缑慧阳研究员及其合作者利用金刚石对顶砧技术对具有线性分子链的二氰乙炔—C4N2分子晶体进行了高压研究,首次澄清了其振动模式的研究中长期存在的争议。并且该研究团队发现C4N2分子晶体在较低的压力下就发生聚合反应,离散的线性分子链在压力驱动下逐渐形成无序的类石墨装的碳氮原子环状结构,并且仍然维持较高的N含量。研究结果发表在近期的Chem. Mater. (DOI: 10.1021/acs.chemmater.7b01446)上。
