Pressure Induced Topochemical [4 + 2] Dehydro-Diels−Alder (DDA) Reaction- Yapei Li, Drs. Haiyan Zheng and Kuo Li
JULY 23, 2021
New work from a team of scientists led by Dr. Haiyan Zheng and Dr. Kuo Li from HPSTAR found the ethynylphenyl of 1,3,5-triethynylbenzene (TEB) undergoes [4 + 2] dehydro-Diels−Alder (DDA) reaction with phenyl at 4 GPa. Their study suggested that the DDA reaction between ethynylphenyl and phenyl is a promising route to decrease the reaction pressure of aromatics, which allows the scalable high-pressure synthesis of nanoribbon materials. The result is published recently in the Journal of Physical Chemistry Letters.
Pressure-induced polymerization (PIP) of aromatics has been a robust method to synthesize novel carbon materials. However, the required polymerization pressures are typically up to ~20 GPa, which is too high to be applied for large-scale preparation and thus prevent its further application.
In comparison, alkynes often polymerize in a solid at much lower pressure (around 4 GPa). And very recently, Zheng’s group also found that the phenyl of diphenylbutadiyne reacted with ethynylphenyl at ∼10 GPa via a [4 + 2] dehydro-Diels−Alder (DDA) reaction — a main reaction in organic chemistry, and produced crystallinegraphitic nanoribbon. So what if there is both phenyl and ethynyl in an aromatic under compression?
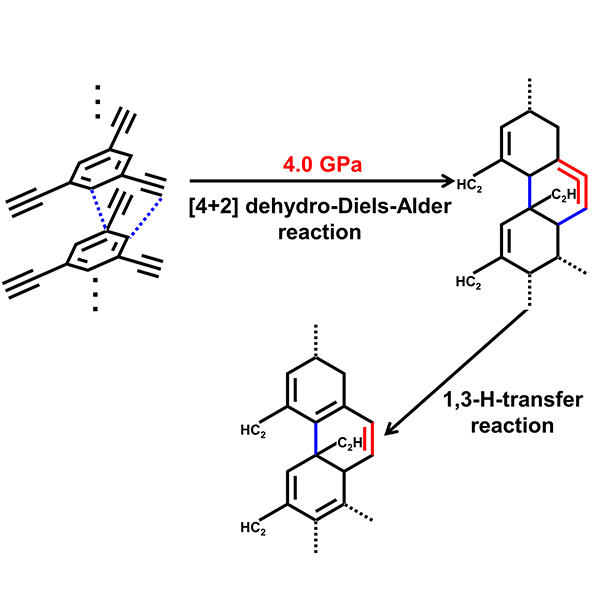
In the current research, by introducing ethynyl to phenyl, the team found the formation of 1-D nanoribbon at 4 GPa from the reaction between ethynyl and phenyl in TEB . They also found that the phenyl and ethynyl reacted simultaneously at 4 GPa to produce sp3 C−H and C=C from Raman spectrospy. And the reaction in TEB was irreversible with a typical intermolecular C···C distance above 3.3 Å — larger than the critical PIP distance of 2.8 Å for aromatics and 2.9−3.1 Å for alkyne, from X-ray diffraction measurements..
Their further meta-dynamic calculation (M-D) at 4 GPa and 300 K show a synergetic bonding process between ethynylphenyl and ethynyl, which suggest the reaction in TEB could be a [4 + 2] DDA reaction . So the team proposed several product models and used diverse cutting-edge techniques including Raman, IR, lab XRD, TEM, SAED, solid-state NMR to investigate the product and confirmed that the products were crystalline sp2−sp3 1-D nanoribbon.
"Our study demonstrated that the synergetic reaction of ethynyl and phenyl can help to decrease the polymerization pressure and thus improve the crystallinity of the polymerized product efficiently, which provides an example for high pressure large-scale synthesis of 1-D carbon nanoribbons,” said Dr. Zheng.
Caption: Pressure induced topochemical [4 + 2] Dehydro-Diels−Alder (DDA) reaction in TEB.
压力诱导芳烃的聚合是一种合成新型碳材料的有效方法。然而,芳烃聚合所需的聚合压力一般高达20 GPa左右,此压力不利于大规模合成以及应用。相比之下,炔烃通常在更低的压力下(约4 GPa)聚合。并且, 北京高压科学研究中心的郑海燕,李阔课题组最近的研究发现1,4-二苯基丁二炔分子在10 GPa通过[4 + 2] 脱氢狄尔斯—阿尔德反应并产生结晶的纳米石墨带。那么如果苯基和炔基同时存在的聚合反应值会发生什么样的新奇变化呢。本研究中,该研究小组通过压力诱导1,3,5-三乙炔基苯分子晶体发生聚合并得到了sp2-sp3杂化的一维晶态碳纳米带。他们发现1,3,5-三乙炔基苯分子在高压下主要发生的是脱氢狄尔斯—阿尔德(Dehydro-Diels-Alder(DDA))反应。原位拉曼和红外光谱确定了反应压力为4 GPa, 并且原位X-射线衍射确定了临界反应压力(3.6 GPa)下的晶体结构和此压力下苯基与炔基的DDA反应的临界距离为3.3 Å。他们同时综合利用离位拉曼,红外光谱,X-射线衍射,透射电镜、固体核磁等一系列表征手段并结合理论计算详细分析了反应产物的结构,确定了苯基和炔基在高压下具有协同效应,在4 GPa时发生了[4+2]脱氢狄尔斯—阿尔德反应,且主产物为sp2-sp3杂化的一维晶态碳纳米带。该研究表明,乙基和苯基的协同反应有助于降低聚合压力,从而有效地提高聚合产物的结晶度,这为高压大规模合成一维碳纳米带提供了思路。相关结果发表在近期的Journal of Physical Chemistry Letters (DOI: 10.1021/acs.jpclett.1c01945)上,文章第一作者为北京高压科学研究中心博士生李亚培。
