Pressure induced topochemical Dehydro-Diels-Alder reaction – Drs. Kuo Li, Haiyan Zheng and Peijie Zhang
SEPTEMBER 28, 2020
New work from a team of scientists led by Drs. Kuo Li and Haiyan Zheng from HPSTAR collaborated with Dr. Jing Ju from Peking University found pressure-induced polymerization of 1,4-diphenylbutadiyne (DPB) produces crystalline graphitic nanoribbons. Their study shows the topochemical reaction route of DPB starts from the Dehydro-Diels-Alder (DDA) reaction instead of the traditional 1, 4-addition reaction. The result is published recently in Journal of the American Chemical Society.
Solid-state topochemical polymerization (SSTP) is a process whereby the confinement and preorganization of the crystal forces a chemical reaction to proceed with a minimum amount of atomic and molecular movement under external physical stimuli (light, heat, pressure, etc.). This specific feature helps the reaction to generate crystalline and ordered products that are structurally related to the reactants. Unfortunately, the reaction types of the SSTP are limited to a few types (such as 1,4-addition, [2+2] cycloaddition and azide-alkyne cycloaddition). Diels-Alder (DA) reaction is a textbook cyclization reaction between a diene and a dienophile. If at least one carbon-carbon double bond is replaced by a carbon-carbon triple bond in the DA reaction, the reaction is called a Dehydro-Diels-Alder (DDA) reaction. The DA reaction and the DDA reaction are widely used for building a new six-membered carbocycle in solution, but are scarcely seen in solid-state reaction, because achieving the proper orientation and distance between a diene and a dienophile is extremely challenging.
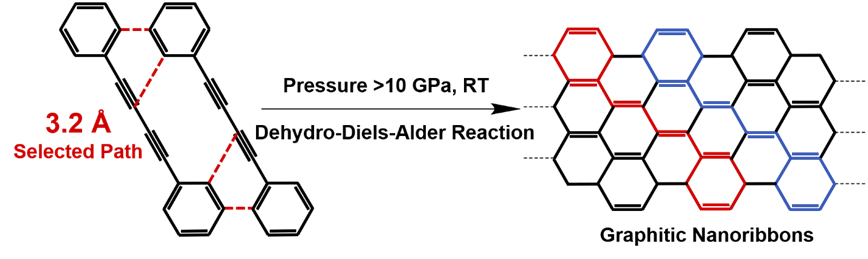
Applying high pressure is proven to be an effective way to regulate molecular stacking, compress the intermolecular distance, and thus induce the topochemical reaction in a constrained crystallized environment. By using in situ Raman and IR spectroscopy, the authors found that the SSTP of DPB under high pressure starts via an unexpected DDA reaction with phenyl as dienophile instead of 1,4-addition reaction between diynes. In situ high-pressure neutron diffraction was used to explore the crystal structure of DPB at the reaction threshold pressure (10 GPa) and the critical distance of this DDA reaction was determined as 3.2 Å. Furthermore, the authors also used multiple cutting-edge techniques including IR, SEM, TEM, XRD, SAED, neutron PDF, solid-state NMR and DFT calculation to investigate the product and confirmed that the products are crystalline armchair graphitic nanoribbons. Finally, based on several quantitative distances of the different reactive positions before reaction, they proposed that the SSTP is dominated by the distance of reactive positions, which is different from the solution reaction dominated by the active of functional groups.
拓扑化学反应主要指由外界物理刺激(光,热,压力等)诱导发生在晶体中的化学反应。拓扑化学反应可以最大限度的保留产物结构与反应物结构的关联性,因此是构建晶态聚合物的一种有效方法,然而,常压下该类反应的类型十分有限,因此限制了拓扑化学合成方法的进一步发展。压力是调控分子堆积方式及分子间距离的有效手段,几乎可以使所有的不饱和有机小分子发生聚合,因此是开发新的拓扑化学反应类型的主要途径之一。北京高压科学研究中心的郑海燕、李阔课题组与北京大学的鞠晶团队合作通过压力诱导1,4-二苯基丁二炔分子晶体发生聚合得到了晶态的纳米石墨带。进一步的研究表明1,4-二苯基丁二炔分子在高压下主要发生的是脱氢狄尔斯—阿尔德(Dehydro-Diels-Alder(DDA))反应而非传统的1,4-加成反应。研究者利用原位高压中子衍射研究了临界反应压力(10GPa)下的晶体结构,并确定了苯乙炔基与苯基的DDA反应的临界距离为3.2 Å。他们同时综合利用X-射线衍射,红外光谱、扫描电镜、透射电镜、固体核磁、原子对分布函数等一系列表征手段并结合理论计算详细分析了反应产物的结构,确定了产物为两种不同氢含量的纳米石墨带。此外,研究者进一步比较了临界反应压力下1,4-二苯基丁二炔分子晶体中其它可能路径的反应距离,提出拓扑化学反应是由“距离选择”所主导,与由官能团活性选择所主导的溶液反应不同。该研究将脱氢狄尔斯—阿尔德(Dehydro-Diels-Alder(DDA))反应引入到拓扑化学反应中,同时为晶态纳米石墨带的合成提供了一种新的可控的“自下而上”的合成策略。该结果发表在近期的Journal of the American Chemical Society (DOI: 10.1021/jacs.0c08274)上,文章第一作者为北京高压科学研究中心博士生张沛捷。
