Counterintuitive phase behaviour observed in isotopic hydrogen (H2-HD-D2) alloys - Drs. Philip Dalladay-Simpson and Ross Howie
JUNE 02, 2020
In an international collaboration of scientists based at HPSTAR, Hefei institute of Solid-state physics and the University of Edinburgh, the phase diagram of the fundamental ternary molecular alloy, bearing isotopes: hydrogen (H2) deuterium (D2) and hydrogen deuteride (HD), was carried out to the most extreme conditions, up to 200 GPa and down to 13 K. These extensive measurements, which are the first of their kind and published in a recent issue of PNAS, implemented in-situ high-pressure low-temperature Raman spectroscopy to identify and constrain phase transitions within these alloys over broad pressure-temperature-compositional regimes. The resulting observations were found to be classically counterintuitive, finding that mixtures of H2, HD and D2 behave as an isotopic molecular alloy (ideal solution), and exhibit symmetry-breaking phase transitions between phases I-II, I-III and II-III. Remarkably, all transitions occur simultaneously amongst the isotopes and at higher pressures for the alloys than either pure H2 or D2. This runs counter to any quantum effects based on isotope mass but can be explained by quantum trapping of high kinetic energy states by the exchange interaction.
Rich phase diagrams are inherent to molecular systems, where the interplay of pressure, temperature and isotopic mass can modify the present intermolecular interactions. This can be manifest as solid-solid phase transitions due to the complete or partial inhibition of molecular dynamics (rotations, vibrations etc.) or even perhaps in the most extreme situation, the degeneration of the molecular state itself. In addition to the classical parameters (pressure, temperature and mass) the phase diagrams of the hydrogens, H2, HD and D2, are heavily influenced by quantum principles owed to the effects of exchange symmetry, nuclear spin statistics and a significant zero-point energy, resulting in fascinating behaviour at low temperatures.
Molecular hydrogen forms the archetypical quantum solid. Its quantum nature is revealed by behaviour which cannot be explained only through classical means. Further, hydrogen and its heavier isotope, deuterium, have unique and distinctive properties which set them aside from the rest of the periodic table. Hydrogen has the lowest nuclear mass, and H2:D2 has the highest isotope mass ratio of any element 1:2. Consequently, quantum effects such as zero-point energy (kinetic energy) are both large and different for the two isotopes. This energy, combined with the effects of exchange symmetry, via the para-ortho distinction, result in fascinating behaviour at low temperatures. When two isotopes are mixed, they spontaneously form yet another isotope hydrogen deuterium (HD). Isotope effects between H2, D2 and HD molecules come from mass difference, and the different quantum exchange effects: fermionic H2 molecules have antisymmetric wavefunctions, whilst bosonic D2 molecules have symmetric wavefunctions and HD molecules have no exchange symmetry.
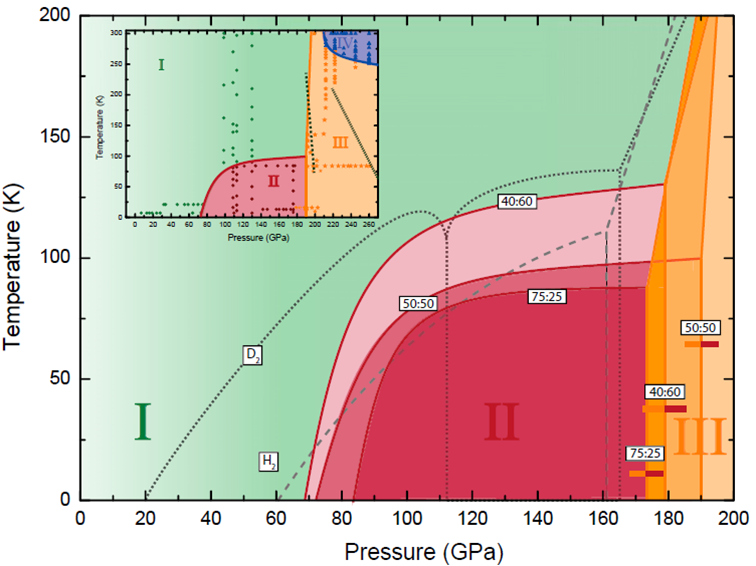
The study, which compressed alloys, consisting of H2-HD-D2 molecules, at low temperatures, 13-300 K, reports phase transitions having peculiar characteristics, requiring higher and/or lower temperatures in comparison with either the pure H2 or D2 counterparts. “Since HD has an intermediate mass and prevalent component in these alloys, one would expect with its addition phase transitions would occur at intermediate P-T regimes,” said lead scientist Eugene Gregoryanz. The discrepancy from the more classical understanding of molecular phase diagrams, derives from the quantum nature of the hydrogen molecules themselves, where the exchange-symmetry can in effect trap the molecules in different, higher energy states.
Caption: Proposed low-temperature phase diagrams of three representative H2–D2 mixtures (H2:D2 ratios of 75:25 = 3:1, 50:50 = 1:1, and 40:60 = 2:3).
氢-氘是高压下非常有趣的体系,我们关于氢和氘已经进行了一系列的研究,然而还有很多重要的问题尚待解答:由于同位素及其核量子效应,氢与氘形成的氢氘(HD)分子是否会在某一条件下分解?是否有区别于纯氢和纯氘的新相的出现?氘的掺杂是如何影响氢的相变等等?近日,北京高压科学研究中心与中国科学院固体物理研究所、爱丁堡大学的合作研究发现:不同比例的氢与氘(氢的同位素)混合物在高压低温下可形成一系列分子固溶体。虽然相对于纯的氢和氘,固溶体也经历了类似的高压结构相变,但相变压力显著提高,揭示了在氢体系中独特的量子效应。相关研究成果以“Counterintuitive effects of isotopic doping on the phase diagram of H2–HD–D2 molecular alloy”为题发表于《美国科学院院刊》。
